APTIVA 4 & SPEEDER WH
FREMS™: Frequency Rhytmic Electrical Modulation System
Electroceutical is a recently coined term that substantially contains all the bioelectronic medicine that employs electrical stimulation to influence and modify the functions of the body, by administering electrical impulses directed to specific nerve fibers or to particular cerebral circuits, which allow the treatment of various pathologies through the autogenous production of neurotransmitters.
The nervous system supervises all the functions of the organism, sending orders through electric impulses.
When these impulses do not work as they should, they could be corrected externally by artificial electrostimulation.
Each tissue/cell has its own specific mode of response to electrical stimuli. Through a stimulation with certain characteristics of frequency, intensity and duration, the excitability of the cell/tissue is activated, generating the “reparatory” response of the organism.
FREMS™ Technology
Covered by several international patents, it is the result of research dedicated to the creation of a non-pharmacological system for the treatment of diseases and complications affecting the peripheral, vascular, locomotor and nervous systems.
It consists of biocompatible electrical signals generated by computerized neurostimulators and administered through transcutaneous electrodes.
The FREMS™ signals are biocompatible because, unlike traditional electrotherapies, they are simultaneously modulated in frequency, amplitude and duration, depending on the biological tissues that it desires to interact with, producing a rapid and lasting therapeutic and antalgic effect.
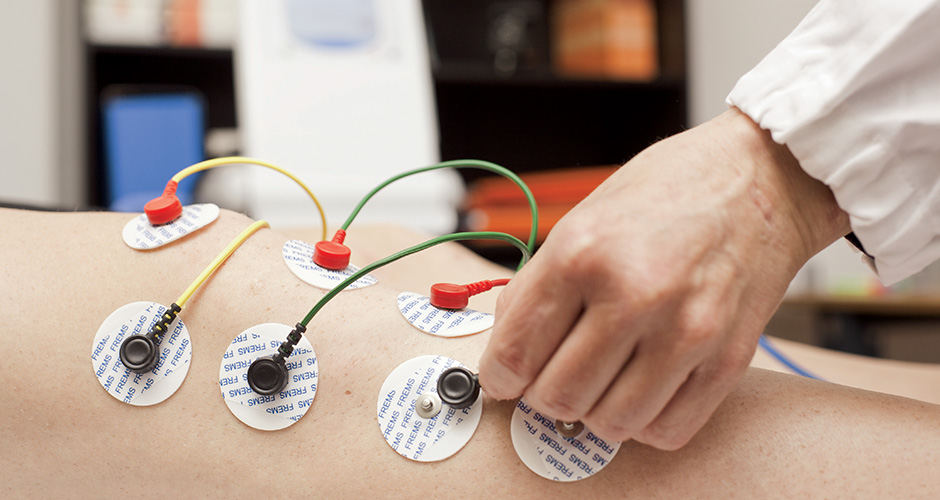

Clinical efficacy
FREMS™ promotes a lasting healing effect and rapid pain reduction without side effects.
To date, some mechanisms of action and biological effects on our organism have been scientifically demonstrated, promoted by specific sequences:
- Vasomotor reactivation of the microcirculation
- H-reflex Modulation
Frequency rhythmic electrical modulation system (FREMS) on H-reflex amplitudes in healthy subjects
- Increase in the microcirculation activity
- Increase in the production and release of growth factors such as VEGF and bFGF (neoangiogenesis)
- Interaction with neuromuscular transmission
Frequency rhythmic electrical modulation system (FREMS) on H-reflex amplitudes in healthy subjects
A randomized controlled study on the effect of two different treatments (FREMS AND TENS) in myofascial pain syndrome- Pain reduction over time
- Increase in the vasculature in the treated area
Electrical stimulation as adjuvant treatment for chronic leg ulcers of different aetiology: an RCT
Effectiveness of the frequency rhythmic electrical modulation system for the treatment of chronic and painful venous leg ulcers in older adults Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers
- Decontracting activity
Frequency rhythmic electrical modulation system (FREMS) on H-reflex amplitudes in healthy subjects
- Anti-inflammatory and draining effect
Therapeutic effects
FREMS™ is proposed as a valid and innovative approach in the care of:
musculoskeletal disorders such as radiculopathy (cervicobrachialgia, low back pain, carpal tunnel, etc ...);
sports injuries (muscle injuries, tendinopathies, etc.);
peripheral neurovascular complications such as Painful Diabetic Neuropathy, Vasculopathies (arteriopathies, venous or mixed stasis), Chronic ulcers of various etiology.
The ideal solution
Long-lasting functional recovery and a rapid analgesic effect.
It integrates perfectly with other rehabilitation systems offering a valid alternative to pharmacological treatment and delaying / avoiding any surgical intervention.
Excellent results with patients refractory to other therapies or treatments.
Ideal for treating acute traumas.
The only scientifically validated neurostimulation to exert an action of neo-angiogenesis by promoting the release and synthesis of VEGF and other growth factors.
Innovative, patented and certified system.
Easy to use.


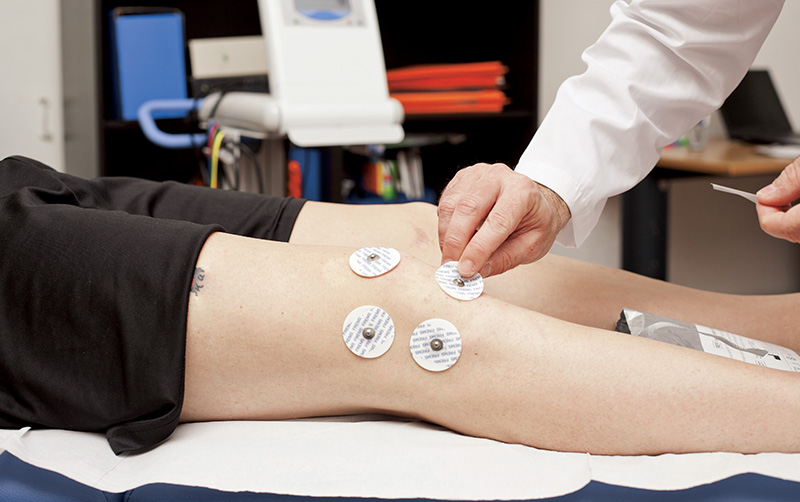

The application
The FREMS™ therapy is administered by the Aptiva Fisio Frems, Aptiva 4 medical devices and their respective portable Speeder models, applying small disposable transcutaneous electrodes certified specifically for the FREMS™ application on the area to be treated. The electrodes must be positioned according to standards defined for each specific treatment and protocols tested for each individual application.
The treatment consists of a cycle of sessions of about 25 minutes whose number is determined by specific protocols for each type of pathology, allowing the choice between acute, sub-acute and chronic. The specific treatment program and the number of sessions is established by the doctor.
The Devices
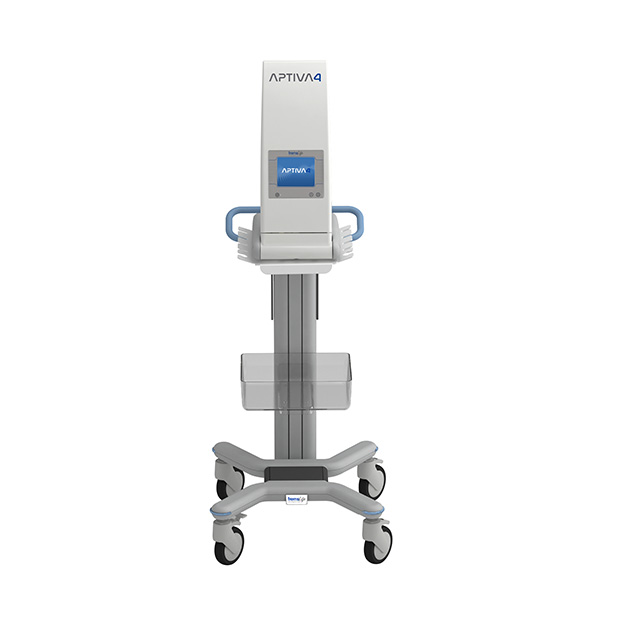
APTIVA 4
The new Medical Device APTIVA 4™ is dedicated to the treatment of ulcers of various etiology and disabling vascular complications such as Painful Diabetic Neuropathies and Vasculopathies.
It represents the evolution of a technology consolidated over time that has already demonstrated its high therapeutic efficacy. With the improvements made, both the simplicity of use and the reliability of the results have increased.
It is equipped with 4 independent neuro stimulation channels each of which has 4 dipoles. The 32 total electrodes therefore allow an adequate differentiated stimulation capacity for over 20 application protocols.
It is also equipped with the electrode detachment control function, which guarantees the best efficacy of each treatment and highlights any anomalies even before starting the therapy. In fact, during the session it is possible to check whether the electrodes transmit the FREMS™ therapy correctly, in order to optimize the final result.
APTIVA 4 is a medical device (class IIa) with the CE and INMETRO mark and with FDA 510 (k)* certification. The equivalent model APTIVA JAZZ, whose sale is limited to Russia, is provided with the GOST R mark.
The Aptiva 4 device can be requested in full version, also including the physical therapy and sport rehabilitation protocols present in the APTIVA FISIO FREMS device. Software, user manuals and application manuals are available in Italian, English, Spanish, Portuguese and Russian.
In order to increase the manageability and practicality of the device, it is possible to request a suitcase for transport and a trolley on which to install the device, thus being able to transport it easily in an outpatient setting and keep the stimulation cables in an orderly manner.
* Limitations of use for the US market. Consult directly with the manufacturer.
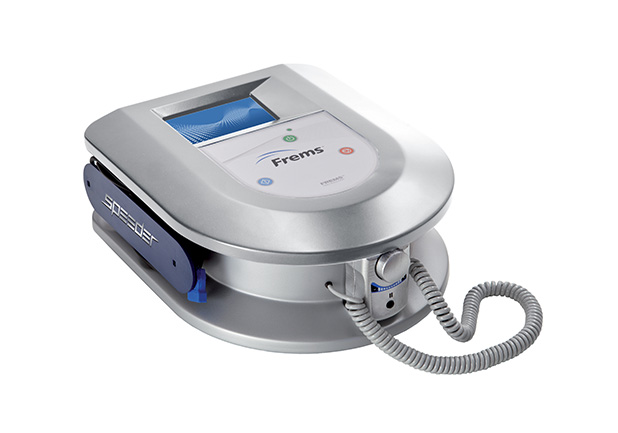
SPEEDER WH
Where you want, when you want
SPEEDER WH is the FREMS ™ tool, ready for use in any situation, thanks to its portability and manageability. It has a compact design and uses a touch screen interface and soft key keyboard.
It is equipped with two stimulation channels (each of which has two dipoles) and uses both single-use and multiple-use electrodes FREMS™ branded.
The software is intuitive and contains protocols for the treatment of cutaneous ulcers and neuropathies
The following classes of patients are excluded from the use of APTIVA 4 and SPEEDER WH:
patients with diagnosed neoplastic disease;
patients with an implantable electronic device (eg pacemaker, defibrillator or neurostimulator);
pregnant women;
patients with previous episodes of epileptic seizures.
The applications

Painful diabetic neuropathies
It is one of the complications of type 2 diabetes (or diabetes mellitus), a condition that damages the ability of nerve fibers to transmit stimuli. This results in sensory and motor disturbances, with the appearance of various symptoms such as cramps, tingling, headaches and pain especially during the night.
Diabetic neuropathy can be expressed with a generalized form, extended to the whole peripheral nervous system both sensory-motor and autosomal, or in a series of single or multifocal clinical forms characterized by the involvement of a single nerve or plexus nerve.
The US National Institute of Diabetes estimates that the percentage of diabetic patients affected by some form of diabetic neuropathy stands at 60-70%.
FREMS™ therapy promotes the improvement of tactile sensitivity in limbs affected by neuropathy and the reduction of painful symptoms both daily and nocturnal, thus allowing a better quality of life, better walking and better nighttime restorations. These results last up to 4 months, after which it is recommended to repeat a course of therapy.
References:
https://www.ncbi.nlm.nih.gov/pubmed/23238789
https://www.ncbi.nlm.nih.gov/pubmed/26391066
https://www.ncbi.nlm.nih.gov/pubmed/20207576
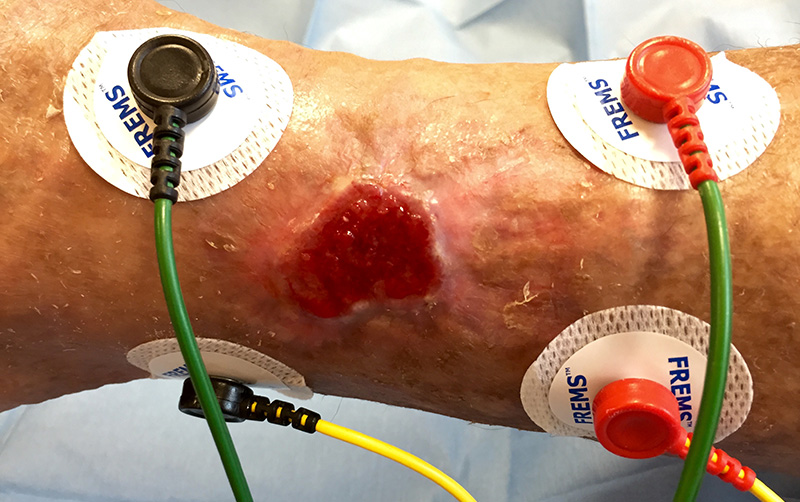
Cutaneous ulcers
Skin ulcers are wounds without a spontaneous tendency to scarring, the consequence of diseases such as vascular diseases, diabetes and diseases that cause patients to be become bedridden.
In Italy, chronic skin lesions afflict more than 2 million people, including 30,000 children, more than 10 million in Europe. It is estimated that around 80 million of the world’s population is affected (Data WUWHS).
FREMS™ Therapy is effective in the treatment of cutaneous ulcers of various etiology, as it reduces pain (in the presence of painful symptoms) and promotes granulation and re-epithelialization of tissues by increasing the synthesis of vascular tissue growth factors.
References:
https://www.ncbi.nlm.nih.gov/pubmed/26391066
https://www.ncbi.nlm.nih.gov/pubmed/20207576
https://www.ncbi.nlm.nih.gov/pubmed/18629524
Vasculopathies
Peripheral vasculopathy indicates a series of general pathological conditions characterized by the appearance of occlusive – thrombotic alterations at the arterial or venous level.
FREMS™ therapy promotes pain reduction, improved oxygenation in the tissues of the treated limbs and an increase in distance covered with absence of pain in vasculopathy with claudicatio. FREMS™ is a valid solution for non-surgical revascularization in patients with technically surgically inoperable ischemies.
References:
Amputation rate reduction using neurostimulation in critical limb ischemia in patients technically inoperable, E. Ricci, G. Garippo, F. Moffa, E. Tonini, B. Bardelli, R. Cassino, EWMA 2009 (European Wound Management Assosiation) Helsinki
FREMS in diabetic foot syndrome, R. Da Ros, 5th IDFS 2009 (Annual Conference of the Israeli Diabetic Foot Study) Tel Aviv, Israel, 2009Are you a physiotherapist and would you like a quote?
Are you a physiotherapist and want to receive information on FremsLife products and stay updated on our training courses, fill out the form below